As a leading specialist diagnostic company, our mission is to deliver improved outcomes for patients in our selected fields of specialty. We deliver innovative and effective assays, and instruments of the highest quality. We actively encourage the discovery of novel biomarkers in our selected specialist fields, and ultimately drive towards their successful commercialization.
The strategic intent of IDS is to hold a leadership position in specialist diagnostics. Our aim is to complement the products supplied by the main diagnostic providers rather than compete with them. We continue to develop and market both manual and fully automated specialist immunoassay testing solutions for both the Research and Clinical laboratory markets.
The increase in the aging population throughout the world has resulted in a higher incidence of conditions such as osteoporosis, rheumatoid and osteoarthritis, renal and cardiovascular disease. The spiralling socio-economic costs of fractures and other age-related morbidities are driving the need for better tools to monitor health and diagnose disease onset much earlier. The goal of IDS is to satisfy this need.
At IDS we continually strive to deliver the highest quality in all aspects of our operations. It is vital that the service we provide to our customers is of the highest standard. A robust and well-structured Technical Service function is essential to maintaining product quality and customer trust. IDS has successfully established a strong technical support function for our range of manual products and IDS-iSYS products .
The IDS Group has 3 manufacturing sites in Europe; in UK, France, and Belgium. Each site has ISO 13485 Medical Device Quality Management System certification All products manufactured by IDS are CE marked, and in addition many of our products, including the IDS-iSYS instrument, the IDS-iSYS Vitamin D assay, and several other IDS-iSYS assays have clearance granted by the US Food and Drugs Administration (FDA) for sale in the USA.
Omicron Pharmaceuticals is the Official Exclusive Distributor for IDS-iSYS in Lebanon and the Middle East.
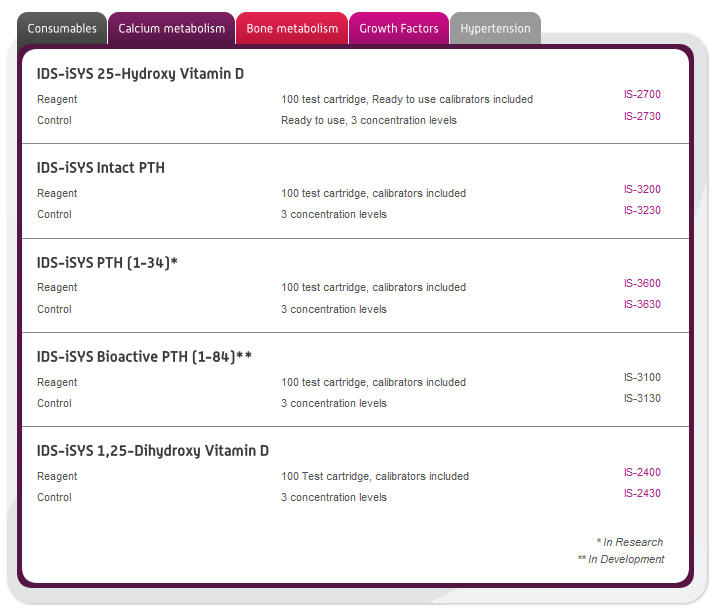
Immunodiagnostic Systems (IDS) is dedicated to the development and provision of innovative immuno assays and automated immunoanalyser technologies for use in clinical and research laboratories worldwide. The company enjoys a dominant position in the provision of immunoassay kits for the determination of Vitamin D (both 25-Hydroxy Vitamin D, and 1,25-Dihydroxy Vitamin D).
The IDS-iSYS Specialty
Immunoassay System
Historically, analytical challenges have made performance of specialty assays impossible for all but the highest volume or most specialized laboratories. Until now.
Designed with the flexibility needed to accommodate unique and challenging assay requirements, IDS-iSYS automation brings testing efficiency and uncompromised quality to specialty immunoassay testing in laboratories of all types and volumes.
Uncompromised Quality
and Efficiency
The IDS-iSYS combines 50 years of instrumentation engineering experience with world-respected assay development skills into a compact, benchtop system that fully automates testing of the most challenging calcium, bone, growth and hypertension markers.
Uncompromised Efficiency
• Full, walk-away automation
• Compact, benchtop design
• Continuous loading with Batch, Random, and STAT flexibility
• Easy operation with auto start-up and shut-down
• On-board refrigeration of ready-to-use reagent cartridges
Uncompromised Quality
• Excellent correlation with gold-standard assays
• Independent test cuvette processing
• Traceability of samples, reagents, and consumables
Designed with the flexibility needed to accommodate unique and challending assay requirements, IDS-iSYS automation brings testing efficiency and uncompromised quality to specialy immunoassay testing in laboratories of all types and volumes.
System
- Multi-discipline Immunoassay System.
Dimensions
- System: L 105 x H 70 x W 75 cm.
- Weight: 103 kg.
- PC: L 60 x H 40 x W 50 cm*.
Throughput
- Up to 120 tests/hour (assay dependent).
Luminometer
- Wavelengths: from 300 to 500 nm.
- Linearity: Up to 10 million RLU.
Spectrophotometer
- Spectrophotometer with interferential filters.
- 6 wavelengths available: 340, 405, 500, 540, 580 and 620 nm.
- Halogen lamp: transmission by fibre optic.
- Linearity: Up to 0 to 3 OD.
- Optic path of cuvettes: 0.8 cm.
Samples
- Serum, plasma or urine.
- Primary Tubes: 4.5 mL, 6 mL, 8.5 mL, 10 mL.
- Secondary Tube: 5 mL.
- Micro cups: 500 μL, 2 mL.
- Further sample tubes can be easily configured.
- 120 positions for samples, calibrators and controls(6 racks with 20 positions).
- On-board dilution and automatic pre-treatment.
Reagent Module
- Up to 15 different immunoassay cartridges.
- Storage at 12 – 15°C while operating.
- On-board storage between 8 – 10°C in standby mode.
*PC dimensions may vary depending on make and model.
Barcode readers
- Reader on front face of system for barcode identification of cuvettes and ancilliary reagents.
- Integrated reader for automatic reading of reagent cartridge barcodes.
- Integrated reader in sample compartment for automatic reading of barcoded sample tubes (positive identification)
- Barcode types: Codes: 2/5, EAN/UPC, Code 39, Code 128 and Codabar.
Sample Handling
- Single pipetting arm for samples and reagents.
- Liquid level detection by capacitance.
- Internal and external rinsing between each pipetting of sample.
Carousel
- Temperature regulated at 37°C ± 0.5°C
- 90 positions for disposable cuvettes.
- Automatic cuvette supply by cuvette loader.
- 4 Independent washers
Cuvette Loader
- Loader for 1 x cube of cuvettes.
- 1 x cube contains 960 disposable cuvettes.
- Thermostatically controlled
Waste collection
- 10 litre container for liquid waste.
- Solid waste (cuvettes) disposed of in solid waste drawer.
Power supply
- Voltage: 100 – 240 V.
- Frequency: 50 – 60 Hertz.
- Maximum power consumed: 750 VA.
Operating System
- Windows XP Pro Service Pack 2
- Windows Vista Service Pack 1
- Windows 7
- Connection to LIS: bidirectional communication (ASTM protocol).

Our Professional Technical Support team will support you with:
– Frontline telephone assistance
– Online Support Portal (Coming Soon)
– Remote dial-in system diagnostics
– Application training and advice
Technical Support
Lebanon
St. George Street, Gemini bldg, B1,
Baabda,
Beirut – Lebanon
- T: +961 5 922 831
- F: +961 5 922 831
- E: info@omicron-pharma.com
|
10 Must-Ask Questions Do all systems on the market meet the IDS presents 10 must-ask questions before choosing your automated immunoassay system.
Other systems may lose data following a cuvette jam and tests must be repeated.
Other systems may require reagents to be removed, resealed and placed into a refrigerator overnight.
Other systems may have to shut down in the event of washer failure.
Other systems may offer poor traceability of QC results and may not trace ancillaries.
Some suppliers may outsource their instrument manufacturing which gives the supplier less control.
Other systems may be built on old technology and may not offer remote access.
Other systems may not be able to check for actual onboard ancillary levels.
Some systems cannot be operated when the computer is turned off or experiences a failure, causing loss of runs in progress.
The system checks on other systems may be a more time consuming manual process.
Other systems’ software may not track maintenance and will depend on the user to track daily weekly and monthly checks. |

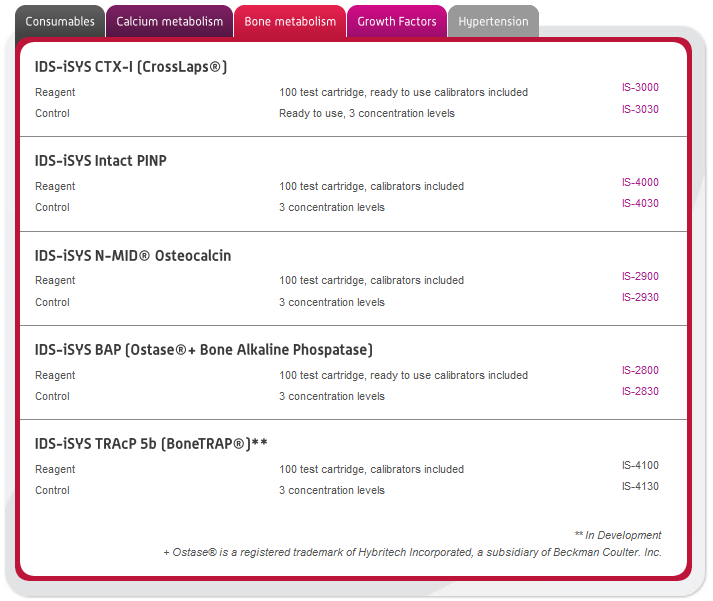
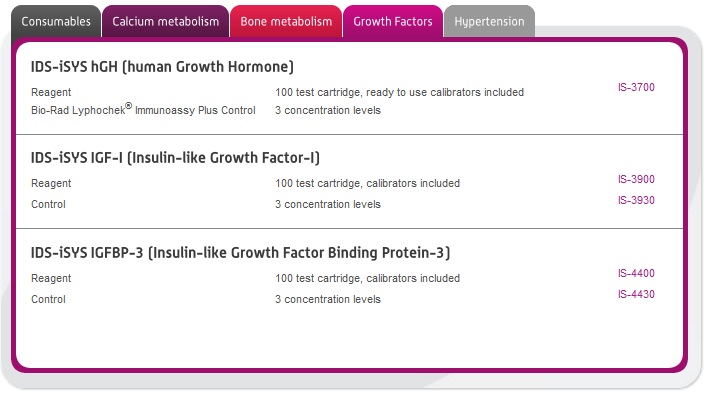
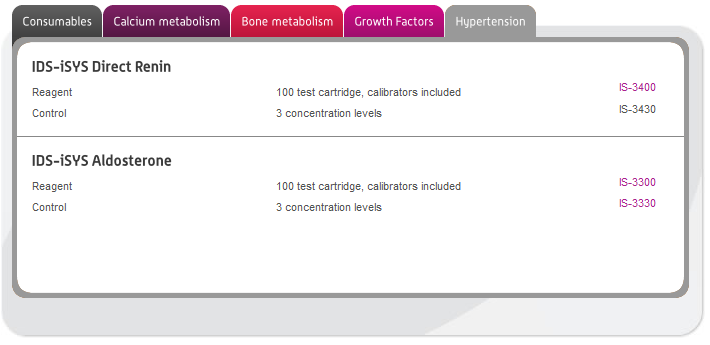
In this section you can browse our large range of manufactured immunodiagnostic kits for use in the following areas:
IDS also distribute a large range of immunodiagnostic kits, antibodies, enzymes and peptides for a diverse range of specialty areas including oncology, inflammation, neuroscience and fertility.
Alternatively, if you are not sure which product you are looking for, please Contact us at: info@omicron-pharma.com